EZ Quality System
The 4-Layer Architectural Hierarchy
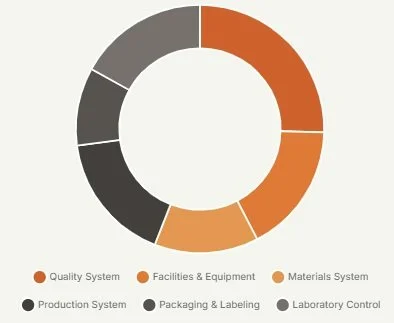
Just as a skyscraper requires a solid foundation, a quality system needs a logical structure. We organize 59 key elements into 4 robust layers, creating a "Granular Control Matrix".
Level 1: Quality Manual
The 'Constitution' of the system. It defines the overall scope and management responsibility. This is the Single Source of Truth governing the entire ecosystem.
Level 2: Policies
Strategic declarations defining 'What we do' to ensure compliance. There are 59 policy documents, one for each key element.
Level 3: SOPs (Rep.+Sub)
Standard Operating Procedures. Includes Representative SOPs (Process Flows) and detailed Sub-SOPs.
Level 4: Records & Evidence
The concrete evidence of execution. Includes Specifications, Validation Protocols, Plans, and Recording Forms.